




Chemistry is central; without bonding we would fall apart...
-Anonymous

-
Metallic bonding is the electrostatic attraction between the positively charged atomic nuclei of metal atoms and the delocalised electrons in the metal. delocalised electrons (•)
Covalent Bonding
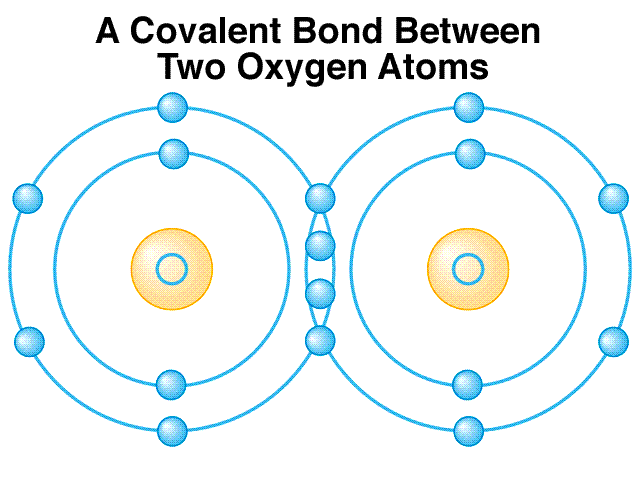
-
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bondingpairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
Ionic Bonding

Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions.

Acids and Bases Nomenclature
Acid names are based on the anion they form when dissolved in water; base names follow the rules for ionic, organic, or molecular compounds.
Balancing Equations

Chemical equations usually do not come already balanced. Making sure they are balanced must be done before the equation can be used in any chemically meaningful way.
All chemical calculations you will see in other units must be done with a balanced equation.