
Covalent Bonding
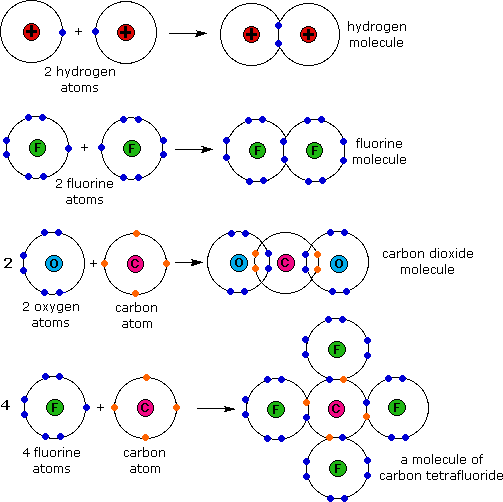
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. By sharing their outer most (valence) electrons, atoms can fill up their outer electron shell and gain stability. Nonmetals will readily form covalent bonds with other nonmetals in order to obtain stability, and can form anywhere between one to three covalent bonds with other nonmetals depending on how many valence electrons they posses. Although it is said that atoms share electrons when they form covalent bonds, they do not usually share the electrons equally.

mucholderthen.tumblr.com

staff.aub.edu.lb
Octet Rule
The Octet Rule requires all atoms in a molecule to have 8 valence electrons--either by sharing, losing or gaining electrons--to become stable. For Covalent bonds, atoms tend to share their electrons with each other to satisfy the Octet Rule. It requires 8 electrons because that is the amount of electrons needed to fill a s- and p- orbital (electron configuration); also known as a noble gas configuration. Each atom wants to become as stable as the noble gases that have their outer valence shell filled because noble gases have a charge of 0. Although it is important to remember the "magic number", 8, note that there are many Octet rule exceptions.
kgortney.pbworks.com
Single Bonds
A single bond is when two electrons--one pair of electrons--are shared between two atoms. It is depicted by a single line between the two atoms. Although this form of bond is weaker and has a smaller density than a double bond and a triple bond, it is the most stable because it has a lower level of reactivity meaning less vulnerability in losing electrons to atoms that want to steal electrons.
chemwiki.ucdavis.edu
Double Bonds
A Double bond is when two atoms share two pairs of electrons with each other. It is depicted by two horizontal lines between two atoms in a molecule. This type of bond is much stronger than a single bond, but less stable; this is due to its greater amount of reactivity compared to a single bond.

chemistry.tutorvista.com
Triple Bonds
A Triple bond is when three pairs of electrons are shared between two atoms in a molecule. It is the least stable out of the three general types of covalent bonds. It is very vulnerable to electron thieves!
